ProLint is intended to be used by both computational scientist and experimentalists alike. Therefore, this tutorial is organized in two parts. The first part explains how you can submit output files from Molecular Dynamics Simulations to the server to calculate the lipid-protein interaction profile of your system of interest. The second part details how you can view, interact and interpret the output results of a ProLint run.
I. Submitting Jobs to ProLint
For testing purposes, we provide ready to use files that can be uploaded to ProLint to see how the webserver
works and to know the type of files that are accepted (see below for more information on file formats). Head
over to the following
page and download some sample
data. There are a few possible system setups available. You can use any system you prefer for this tutorial.
You can also feel free to use any of your own data files as well.
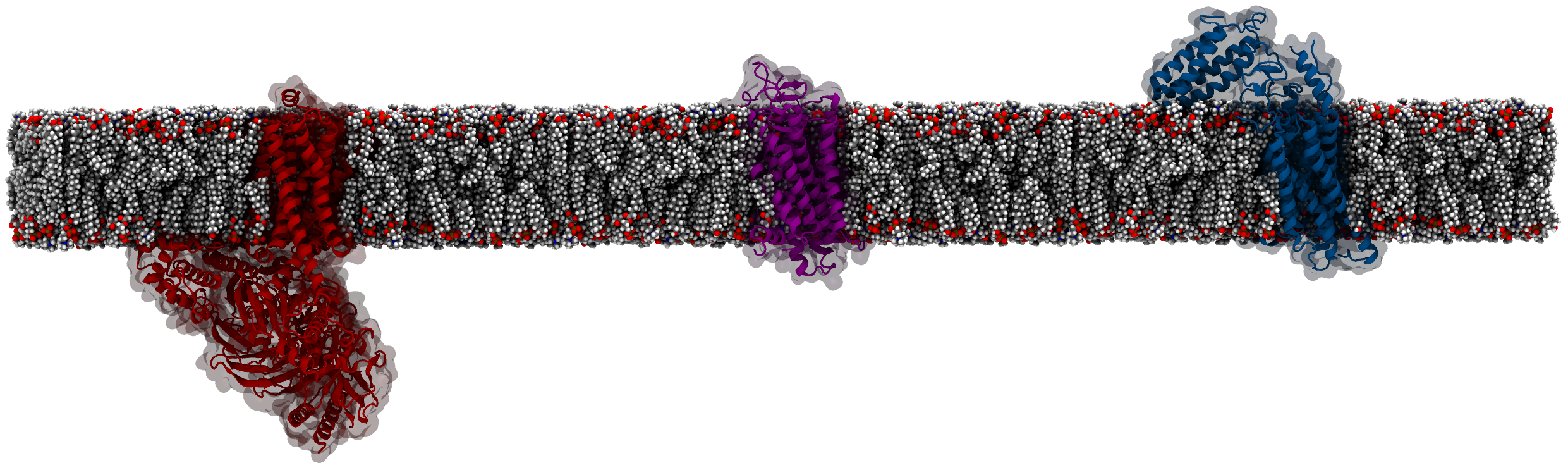
Let's say we want to study the lipid interaction profile of the Smoothened receptor.
From the GitHub repository mentioned above, download the following files (go to the Smoothened folder and
choose the files listed below from
either the mid or small folder; choose the former if you want the calculations to finish quicker or the latter
if you want a better
accuracy of the results.):
- trajectory.xtc
- coordinates.gro
These are simulation files that are generated by GROMACS. The second is a coordinate file (not much different from a pdb file) and the first is a trajectory file that contains information on the dynamics of the system as a function of time. The table below explains the submission form used by ProLint to gather user input. It also gives a description of each field and two different examples of the type of input that is accepted.
| Form Field | Requirement | Description | Example Input 1 | Example Input 2 |
|---|---|---|---|---|
| Submission Title | required | The title of your submission project. Use whatever title you prefer. | My descriptive title! | ABCD-Lipid Interactions |
| Protein Name(s) | required | Give a name to your protein. When multiple protein are present in the system, name them in the order they appear in the topology. | 3SN6-POPC | Protein1, Protein2 |
| Trajectory File | required | Specify the trajectory file to use. | trajectroy.xtc | -- |
| Coordinate File | required | Specify the coordinate file to use. | coordinates.gro | -- |
| Lipid Grouping | optional | Group lipids according to their headgroup type. E.g., POPC, POPE, PIPC, and PIPE lipids would be grouped as PC and PE lipids. For Martini systems you would want to define a larger cutoff compared to atomistic systems. | Check | Uncheck |
| Distance Cutoff | required | The is the distance value that is used to define what constitutes as a contact between lipids and residues. | 7Å | 3Å and 4Å |
| Lipid Filtering | optional | Calcualte interactions only with the listed lipids. | CHOL | CHOL, POP2, PIPC |
| The Resolution | required | The resolution of the input data: martini CG or atomistic | The Martini Model | Atomistic Models |
| Type of Analysis | required | Specify what type of analysis to perform. Contact-based are most time-consuming. | Tick all | Physical-properties |
For the purpose of this tutorial, after downloading the example data mentioned above, you can provide the following input to the form, but please feel free to deviate from the suggestions below:
- Submission Title:
- Protein Name(s):
- Trajectory File:
- Coordinate File:
- Lipid Grouping:
- Distance Cutoff:
- Lipid Filtering:
- The Resolution:
- Type of analysis:
- ProLint Example Project
- SMO
- trajectroy.xtc
- coordinates.gro
- Check
- 5Å
- CHOL, POP1, POP2, POP3, DPG1, DXG1, PNG1, XNG1, DPG3, DXG3, PNG3, XNG3
- The Martini model
- Select any (or a combination) of the options
Above we instruct ProLint to calculate lipid-protein interactions for Smoothened by measuring contacts within a 5Å radius from each residue and only looking for the listed lipids above (and not all 60+ lipid types present in the system, which would have taken conserably more time to compute). We also make sure to check the option to group lipids according to their headgroup type. This allows us to compare the interactions between cholesterol, PIP lipids and GM lipids as a whole.
Click Submit and wait for the files to upload.
Depending on the size of the files and your internet connection, it may take some time until the files are
uploaded. Once that happens, you will be presented with a page where you can monitor the status of your job.
More details will be provided there, but please note that you should either bookmark that page or write down the
unique identifier that
will be given to you. You can use that identifier to access your results for 24 hours after your results have
finished calculating.
Calculating lipid-protein interactions is quite time-consuming so please be patient. It can take up anywhere
from 1-3 hourse for the calculations to finish (depending on the input data). We also have limited resources, so
jobs are put into a queue
waiting their turn to get processed. The time quoted above considers only the calculation time, not queueing
time.
Overview of input file requirements
Input Files have to prepared/processed a little before they can be read by ProLint. This preprocessing step which has
to be done by the user, includes tasks that are standard practice in the MD field and there should be plenty of information
and tools available to do this steps.
Here is a summary of the requirements data files have to satisfy:
- Leave only proteins and lipids in the data files. Remove all water, ion, and any ligand residues from both the coordinate and trajectory files. This can be easily done using standard GROMACS utilities such as trjconv and editconf with an appropriate index file.
- If proteins move freely in the x-y dimension, you may want to process the trajectory to center the protein.
- For best results, make sure to also remove the periodicity of the system.
- Most of the testing has been done with data files using the Martini model. Loading atomistic simulation data is allowed and has been added as an early beta feature. We are actively working on improving it. We welcome you to contact us for more information and to help us fix any potential bugs/issues.
To understand how the backend works and how contacts are calculated see the page on Analysis Reference.
To learn how lipid-protein interactions are visualized and the supported user interactivity, see the page on
Visualization Reference.
II. Viewing Results
This section can be completed by anyone, even if you did not follow the tutorial above.
Background
Why would we want to study how Smoothened (SMO) interacts with its surrounding lipid environment? For one, there is considerable
evidence
showing that lipid-protein interactions in general, and interactions with cholesterol in particular are important
for the function of Smoothened. In fact, cholesterol has been observed
to completely enter the 7-helical core of the receptor. How does cholesterol access the inside of the receptor and
how does it modulate its activity? To get a better understanding, MD simulations may be and have been used.
If you followed the tutorial above and your job finished calculating, then you can access it from the job submission page. You can
view the lipid-protein interaction profile of Smoothened using the different interactive applications implemented by ProLint. If you
did not follow or complete the tutorial, you can still visualize the results of a similar submission (using similar files) that has
been precomputed.
You can access it here .
Conclusions
There are quite a few different conclusions that can be reached from the data. Here is what I believe is an obvious result. We can see that Smoothened forms a highly preferential interaction with cholesterol that is maintained for most of the simulation time. We can also see (e.g., using the Heatmap Viewer application) that this site is located between TM helices II and III of Smoothened. This is a very nice result and it matches quite nicely with what has been previously reported in the literture (for example, here and here).